Abstract
Background: Treatment for relapsed multiple myeloma (MM) has evolved with the availability of many novel agents; outcomes in patients with newly diagnosed MM have improved with IMiD® agents (lenalidomide [R] or pomalidomide) and proteasome inhibitor (PI) use. Regardless of autologous stem cell transplant, many patients receive R maintenance therapy (Jagannath, Blood Adv 2018; Palumbo, N Engl J Med 2012). However, there is a lack of data describing the nature of relapse and treatment choices for patients progressing on R maintenance therapy. Recent phase III data do not evaluate these patients who are relapsed or refractory to R; current treatment guidelines do not specifically recommend second-line (2L) treatment options for these patients (Laubach, Leukemia 2016). However, phase III data show that pomalidomide in combination with bortezomib and dexamethasone improves progression-free survival (PFS) (HR 0.54; P = 0.0027) in R-exposed and refractory patients, including those with 1 prior line (Richardson, J Clin Oncol 2018). The Connect MM Registry is a US, multicenter, prospective observational cohort study designed to examine diagnostic and treatment patterns, clinical outcomes and quality of life in patients with MM. For patients who received R maintenance therapy, we describe patterns of relapse, 2L treatment choice, and outcomes.
Methods: From 250 sites, transplant eligible and ineligible adult patients ≤ 60 days from MM diagnosis were enrolled (N = 3011). Patients were treated at physicians' discretion and followed up for treatment and outcomes until early discontinuation or study end. Patients who relapsed during R maintenance therapy (≤ 15 mg; assessed by treating physician), were analyzed for baseline (BL) characteristics, nature of relapse, and 2L treatment. Patients with symptoms at time of relapse were defined as the presence of ≥ 1 CRAB (hyperCalcemia, Renal failure, Anemia, and Bone disease) criterion ± 60 d from PD; absence of CRAB criteria at PD in this window was considered nonsymptomatic relapse. Primary endpoint was PFS from start of 2L; safety (serious adverse events; SAE) from start of 2L was also assessed. Logistic regression analyses on all BL factors and factors before start of 2L treatment were performed to determine covariates differentiating comparator groups. Survival outcomes were analyzed using a Cox regression after adjusting for covariates.
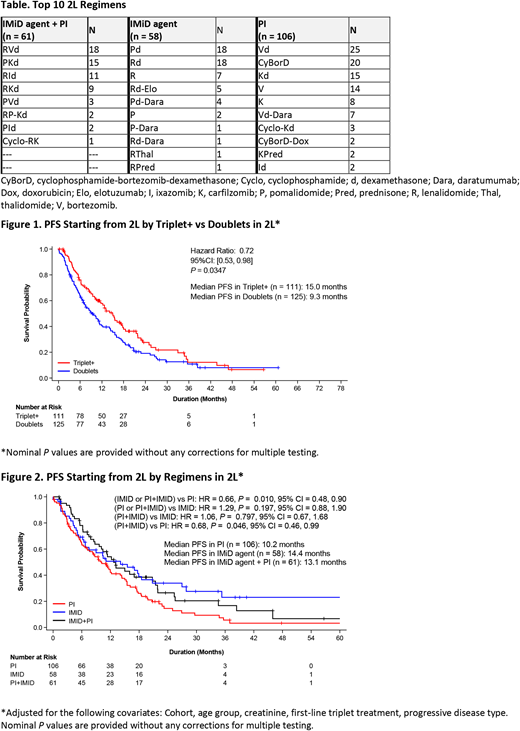
Results: Data cutoff: 1/15/18. Of 2938 treated patients, 1102 entered 2L, 236 having received R maintenance therapy in 1L. Of those patients, 52% (n = 123) and 47% (n = 112) experienced symptoms at time of relapse and nonsymptomatic relapse, respectively (data not available for 1 patient). The top ten 2L regimens are presented in the Table. Median duration of prior maintenance therapy (367 d [IQR: 577] vs 489 d [IQR: 599]) and time from first progression to start of 2L treatment (0.3 vs 0.3 mo) did not notably differ between nature of relapse groups. Prolonged median PFS from start of 2L was observed for patients receiving triplet+ (≥ 3 agents) vs doublet (≤ 2 agents) regimens (HR: 0.72; Figure 1). PFS was better for IMiD agent + PI group compared with PI (without IMiD agents) group (HR, 0.68; Figure 2). Results of sensitivity analyses and overall survival will be presented. SAEs occurred similarly among treatment groups: 42.6% with IMiD agent + PI, 34.5% with IMiD agent (without PI), and 35.8% with PI.
Conclusions: This is the first description of relapse patterns, 2L treatment choice and survival outcomes after PD on R maintenance therapy in community-based patients. Nearly equal numbers of patients had symptoms at time of relapse and nonsymptomatic relapse on R maintenance therapy. After PD on R maintenance, approximately 50% of patients switched to PI regimens without IMiD agents, 25% continued with IMiD agent + PI regimens, and 25% continued in the IMiD agents group. Our data show PFS benefit for triplet+ over doublet treatment in 2L. Patients in the IMiD agent + PI group gained survival benefits over patients in the PI group. Further characterization of the patients continuing with IMiD treatment in 2L after R maintenance therapy relapse (~ 25% of patients) are ongoing. Follow-up with recently approved agents (elotuzumab, daratumumab) and their use in 2L is limited in this analysis. These data may be used to better inform treatment choices after relapse on R maintenance therapy.
Jagannath:Celgene: Consultancy; Merck: Consultancy; Multiple Myeloma Research Foundation: Speakers Bureau; Medicom: Speakers Bureau; Bristol-Myers Squibb: Consultancy; Novartis: Consultancy. Narang:Janssen: Speakers Bureau; Celgene: Consultancy, Speakers Bureau. Ailawadhi:Takeda: Consultancy; Amgen: Consultancy; Janssen: Consultancy; Pharmacyclics: Research Funding; Celgene: Consultancy. Rifkin:Celgene: Consultancy; Boehringer Ingelheim: Consultancy; McKesson: Equity Ownership; EMD Serono: Consultancy; Amgen: Consultancy; Takeda: Consultancy; Sandoz: Consultancy. Terebelo:Celgene: Consultancy; Janssen: Speakers Bureau; Takeda: Speakers Bureau; Pharmacyclics: Speakers Bureau. Toomey:Celgene: Consultancy; Myriad Genetics: Speakers Bureau; Dava Oncology: Other: Travel. Durie:Takeda: Consultancy; Janssen: Consultancy; Celgene: Consultancy; Amgen: Consultancy. Hardin:Celgene: Consultancy. Gasparetto:Janssen: Consultancy, Honoraria, Other: Travel; Bristol-Myers Squibb: Consultancy, Honoraria, Other: Travel; Takeda: Honoraria; Celgene: Consultancy, Honoraria, Other: Travel, Research Funding. Wagner:EveryFit: Consultancy; Gilead: Consultancy; Janssen: Consultancy. Omel:Takeda Oncology: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Srinivasan:Celgene: Employment. Kitali:Celgene: Employment. Agarwal:Celgene Corporation: Employment, Equity Ownership. Abonour:Prothena: Research Funding; Takeda: Consultancy, Research Funding; Celgene: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal